First Trimester Pregnancy - Positive Pregnancy Test
Positive Pregnancy Test?
Congratulations!! You've already made a tiny miracle.
What's next? What should you do to have a healthy pregnancy and minimize risks to both you and your baby?
There is lots and lots of information from many sources including the Royal College of Obstetricians and Gynaecologists RCOG the American College of Obstetricians and Gynecologists ACOG the Perinatal Institute, and the MAMA Academy, among others.
Review blood tests and other tests during your pregnancy here.
In addition to a healthy lifestyle, make sure you are on folate and Vitamin D (see below). Then schedule your appointment with a midwife or obstetrician, and schedule your first ultrasound scan (most women should have 4 scans, and 3 of these are in the first half of pregnancy).
On this page we review:
- Essential vitamins
- Vitamin D
- What a positive pregnancy test means
- Early pregnancy: what we should see by ultrasound
- Miscarriage: 6 things you should know
- Fetal Development
- Nuchal translucency at about 12 weeks and other testing
- Anatomy Scan 18-22 weeks- time for a check head to toe
- The Placenta- the key to 3rd trimester complications
- The cervix -the plug that holds the pregnancy in
- The 3rd Trimester
- Preeclampsia
Essential Vitamins and Check Vitamin D level
Please start folate, and start Vitamin D supplementation if you haven't already and consider checking your Vitamin D level. There are huge short term and long term health benefits for both you and your baby.
Folate (B9), and B12 (which helps folate) are critical for methylation and production of DNA and RNA regulation-- also especially at times of rapid growth during pregnancy. Folate (Vit B9) plays a critically important role in DNA synthesis which is important for both fetal and placental development. Low or deficient levels of folate can lead to many fetal and maternal problems, while folate supplementation has proven to have have these powerful, overwhelming benefits:
- Dramatically lowers the risk of neural tube defects (50-70% reduction)
- Reduces the risk of other cleft type of defects including cleft lip/ palate, and gastroschisis
- Reduces the risk of some heart defects
- Reduces the risk of preeclampsia during pregnancy
- Important for the growth of the placenta, synthesis of DNA and the development of the baby
- Essential for red blood cell production and helps prevent forms of anemia
- Possibly reduce the risk of miscarriage
- Possibly reduces the risk of preterm delivery
Taking folate during your pregnancy is not an option- it's a necessity! It is one of the few things you can do that can actually affect the outcome of your pregnancy!
400 mcg/day of folate is recommended during pregnancy, ideally beginning before conception. It should be noted that the neural tube closes by the 28th day of conceiving and so many females may not even know that they are pregnant before the neural tube normally closes.
Vitamin B12 Aids folate metabolism In Pregnancy
- Essential for baby’s neural tube formation, brain and spine development
- Together with Folate (B9), it works to produce DNA synthesis and red blood cells
- Aids the development and functioning of your brain, nerves and blood cells
- Helps improve your energy, mood and stress levels by aiding the metabolization of fats, carbohydrates, and proteins.
- Helps maintain the normal central nervous system and neurological
Incredibly Important Vitamin D
Vitamin D is really an essential prohormone your body makes every day. But due to modern lifestyle, we don't usually make enough. It plays a critical role in many processes including bone formation, calcium metabolism, placental development controlled placental invasion and brain development. In addition to the effects in adults (see figure right), compelling new evidence strongly indicates that vtamin has D been shown to reduce the risk of a variety of pregnancy related complications, many of which can be traced to placental function.
- prematurity (preterm birth)
- preeclampsia and fetal growth restriction
- gestational diabetes
- possibly reduce the risk of miscarriage
- contributes to the regulation of placental inflammation.
- reduce the risk of autism, both in animal and human studies
- Other positive effects in childhood and adults are reported separately.
Importantly vitamin D therapy has also been shown to improve symptoms in children with autism and reduce the risk of autism. The benefit of vitamin D may be related to the duration of supplementation with some studies showing benefit when vitamin D is started a year before pregnancy.
Most people and all pregnant women should be taking vitamin D supplements.
There is strong and compelling new evidence that all women should have their vitamin D level checked early in pregnancy, and ideally 1-2 more times during pregnancy. The first time should come right after the positive pregnancy test itself.
Vitamin D supplementation is very important for pregnancy, regardless of your level, but your level can determine how much Vitamin D you should take. A good maintenance dose during pregnancy is 1000-2000 IU/ ml but if you are vitamin D deficient, a daily dose of 4000-6000 IU/ ml is recommended. Notice this amount is much higher than you will get with a multivitamin and 10 x the usual recommended dose in non pregnant patients. However, don't be intimidated. Doses up to 10,000 IU/ day are considered safe and there have been no adverse effects associated with this higher dosage. It turns out we have been under-supplementing ourselves for decades and at the same time we've seen autism rates and other conditions increase. Please look, review the data, and decide for yourself. Downloads at bottom of this page.
Gut bacteria and the microbiome may also play an important role in autism.
Vitamins other than the 3 listed here can generally be obtained through diet alone, and vitamin supplementation is not required.
Early Pregnancy: WHAT A POSITIVE PREGNANCY TEST CAN MEAN
A positive pregnancy test is dependent on elevated levels of a hormonal called human chorionic gonadotriopin (HCG). HCG is made by the placental cells which support the embryo- not the embryo itself. This fact is important to remember when we consider HCG levels and early development. A positive pregnancy test tells us that all those complex processes prior to implantation- follicle formation, ovulation, hormonal influence of the micro environment (cervix, cervical mucous, endometrium, and fallopian tubes), and fertilization all occurred perfectly. Implantation has also occurred and we assume the implantation site is safely in the uterus (womb). A sperm contributed by the male has entered the egg produced by the female, and has already made a unique set of DNA- mostly contributed by both parents, but also some unique DNA made by the new individual itself.
Early pregnancy and everything related to it really is a miracle. Humans are very complex organisms, formed in a remarkable short period of time, thanks to our programmed DNA. But because it's so complex, normal human human development doesn't always go perfectly, as we all know. From a practical perspective, whenever a woman becomes pregnant or has a positive pregnancy test, there are 3 primary possibilities (excluding the much likely chance of molar pregnancy which we can consider as a variation of 2 below):
1. Normal pregnancy.
This involves formation of a visible gestational sac by 5 menstrual weeks (3 weeks post conception), a yolk sac, and a living (viable) embryo that should be evident by 6 weeks 4 days (4 weeks 4 days post conception). See below.
2. Non viable pregnancy in the uterus.
Non viable pregnancies inevitably lead to miscarriage. Most miscarriages occur in the first 9 weeks. Remember that non viable pregnancies are typically destined to miscarry based on abnormal fetal chromosomes formed at the time of conception. The pregnancy tries to develop anyway, but fails somewhere along the usual path of development, which inevitably leads to miscarriage. With ultrasound, we might not see anything (if the pregnancy stopped before development of the gestational sac or after miscarriage); an 'empty' gestational sac without yolk sac or embryo; an anembryonic pregnancy with a gestational sac and yolk sac, but no embryo; or early embryonic demise with gestational sac, yolk sac, and demised embryo. We may also see a living embryo which subsequently dies- this is especially likely if the heart rate is unusually low or the sac is very small relative to the size of the embryo. Typically, embryo size also lags behind the true dates.
By the time we see the embryo, we should see a heart beat. Occasionally, we can see a tiny embryo but the heart beat is not definite until a few days later- this only happens with tiny embryos less than 6 mm in size (and usually 2-3 mm). In the case of an 'empty gestational sac' without a detectable embryo, we can rely on internal morphology of the gestational sac, and the size of the gestational sac rather than dates alone. A living embryo is usually seen when the gestational sac is 12-15 mm in mean diameter and should always be seen when the sac reaches 25 mm (in my experience, 20 mm with transvaginal scans). Unless findings are certain, we always assume the pregnancy is potentially viable until proven otherwise, and a followup scan is recommended. Both the embryo and the gestational sac should grow about 1 mm per day, so that a normal, viable pregnancy grows very quickly and is quickly distinguished from a non viable pregnancy. If the pregnancy is not viable, you should remember 6 things below.
A pregnancy that implants outside the uterus, usually in the fallopian tube is the final possibility we consider if the pregnancy test is positive but there is no sign of a pregnancy in the uterus. Ectopic pregnancies cannot grow successfully outside the uterus (with rare exceptions), so these are also not viable. Ultrasound can usually see signs of the ectopic pregnancy, but the pregnancy often does not develop to the stage of a living embryo. More on this in another blog post.
Ultrasound Scans
First Trimester Viability- before 10 weeks
First Trimester Viability- before 10 weeks
First Trimester Viability- before 10 weeks
Most women will have at least 4 scans in the U.S. and 2 in the UK through the NHS. The first viability scan is confirm viability and number of embryos (twins?). Ideally 6-10 weeks. Although not standard in the NHS, if you say you are worried or having symptoms you might get one!
3rd Trimester Growth Scan- 28 to 35 weeks
3rd Trimester Growth Scan- 28 to 35 weeks
3rd Trimester Growth Scan- 28 to 35 weeks
3rd trimester growth scans can confirm that growth is normal, and also check blood flow to the placenta if there is a concern. In the first half of pregnancy, babies grow in length but put on little weight. After 24 weeks, babies continue to grow in length at the same rate but now accelerate growth and maturation. A routine 3rd Trimester growth scan is not offered in the NHS, but should be if there is any concern. Review the GAP protocol and see if you should be getting a growth scan.
Normal Early Pregnancy- Viability Scan
What we will see by ultrasound in the First Trimester
1. 3-5 weeks (1-3 weeks post conception). We usually don't see anything initially during a narrow window after implantation but before the gestational sac is large enough to see.
2. By 5 menstrual weeks (3 weeks post conception), the gestational sac is very recognizable by ultrasound. We can start to see the gestational sac a few days earlier, when it is 2-3 mm in diameter, but should always see it by 5 weeks when the sac should be 5 mm.
3. Very soon after seeing the gestational sac, we will see the yolk sac. It helps provide nutrients to the early embryo and is the first site of red blood cell production.
4. By 4 weeks after fertilization (6 menstrual weeks), the tiny developing embryo will be visible with the first heartbeat seen by ultrasound. We should always see a living embryo by 6 weeks 4 days (4 weeks 4 days after conception). However, remember that your dates may not be as correct as you think they are. Even when you are certain, conception can occur several days after sexual intercourse.

First Trimester Pregnancy Complications- Miscarriage
If the Pregnancy is NOT Viable In the First Trimester Also see blog post
6 Things to remember:
1. You are not alone.
Approximately 1 in 5 pregnancies will end in a miscarriage. Younger women should not assume they are protected from miscarriage. They are not, but both miscarriage and chromosome abnormalities are more common as women get older. The process of miscarriage is Mother Nature's way of dealing with unsuccessful (non viable) pregnancies. It's unfortunate it happens at all, but it is a fact of nature. Fortunately, the process of miscarriage usually occurs early in the first 8-9 weeks or pregnancy, and a woman does not usually need to carry the pregnancy to term before realizing the baby has not formed. Late term fetal demise, in the last few weeks of pregnancy, is fortunately much less common. Both early and late fetal death is tragic, but it's natural for the grief to be that much greater with a longer bond. Support groups are available if you'd like to talk about it.
2. Major chromosome abnormalities are by far the most common cause of a miscarriage in the first trimester. The chromosome abnormalities tend to be more severe- and so different than- the chromosome abnormalities we see later in pregnancy, after 10 weeks, when less severe chromosome abnormalities like trisomy 21 (Down syndrome) are more common. Chromosome abnormalities are common in women of any age but increase in frequency with maternal age- based on the fact that women are born with a finite number of potential eggs. This isn't true for the male contribution- sperm are produced constantly in a male's life time.
3. Symptoms vary. Early on, the patient is not aware of any problems and she assumes the pregnancy is developing normally, even though the stage has already been set for an inevitable miscarriage. Later, she will usually develop spotting, initially very light and sporadic, and later it will become heavier and lead to the miscarriage.
4. It will take longer than you think. The process of miscarriage is typically much slower than the process of early pregnancy development, and can last many weeks. Why is this? Miscarriage begins with slowing of growth of the pregnancy, then stopping it and finally reversing it. It requires detaching the intricate growth of placental cells that have grown into the uterus, and then expelling the pregnancy into the uterine cavity.
5. You are not in control. You have no control over the chromosomes that make the developing embryo. Therefore, we have no control over miscarriage, regardless of what you do or don't do.
6. Nothing has changed for your next pregnancy. In general, a miscarriage does not increase the chance of a miscarriage in your next pregnancy just as normal prior deliveries do not protect you from a miscarriage in your next pregnancy. Remember, every pregnancy is unique, with a unique set of DNA. Having said that, if women experience 2 or certainly 3 or more consecutive miscarriages, there may be other underlying causes and this should be investigated.
Remember also that a miscarriage means the pregnancy developed all the way to implantation and early growth. This also means that all of those complex processes- ovulation, conception, transportation down the tube, and implantation worked perfectly. You and your partner made a pregnancy! That means you can do it again. In fact, the odds are you will return with a successful pregnancy within 6 months.
Management- (from TeachMeObgyn.com)
There are three options for the definitive management of miscarriage, which all carry a similar risk of infection.
Regardless of treatment type, if the patient is Rhesus negative and is greater than 12 weeks gestation, they require anti-D prophylaxis. If they are managed surgically, regardless of the gestation, they require anti-D (if RhD-ve).
Conservative (Expectant)
Conservative management allows the products of conception (POC) to pass naturally. Patients should have 24/7 access to gynaecology services during this time.
- Advantages: Can remain at home, no side effects of medication, no anaesthetic or surgical risk.
- Disadvantages: Unpredictable timing, heavy bleeding and pain during passage of POC, chance of being unsuccessful requiring further intervention and need for transfusion.
- Follow-up: Some units will arrange a repeat scan in two weeks. Others will arrange a pregnancy test 3 weeks later.
- Contraindications: Infection, high risk of haemorrhage ie. Coagulopathy, haemodynamic instability.
Medical
Medical management involves the use of vaginal misoprostol (prostaglandin analogue) to stimulate cervical ripening and myometrial contractions. It is usually preceded by mifepristone 24-48 hours prior to administration.
- Advantages: Can be at home if patient desires, with 24/7 access to gynaecology services, avoid anaesthetic and surgical risk.
- Disadvantages: Side effects of medication: vomiting/diarrhoea, heavy bleeding and pain during passage of POC, chance of requiring emergency surgical intervention.
- Follow-up: Pregnancy test 3 weeks later
Surgical
Surgical management involves a manual vacuum aspiration with local anaesthetic if <12 weeks, or evacuation of retained products of conception (ERPC).
In ERPC, the patient is under a general anaesthetic, a speculum is passed to visualize the cervix, it is dilated allowing suction tube to be passed and remove the products of conception. Patients typically attend hospital on the day of the procedure and are discharged the same day
- Definite indication: Haemodynamically unstable, infected tissue, gestational trophoblastic disease.
- Advantages: Planned procedure (may help patient to cope with miscarriage), unaware during the process (patient under general anaesthetic).
- Disadvantages: Anaesthetic risk, infection (endometeritis), uterine perforation, haemorrhage, Ashermen’s syndrome, bowel or bladder damage, retained products of conception.
Early Fetal Development
By 10 weeks after fertilization (12 menstrual weeks), the fetus is mostly formed with very recognizable parts- the brain, spine, heart, face, lungs, abdomen, stomach, urinary bladder, arms, legs, hands, feet, fingers, and toes.
We are incredibly fortunate to share this miracle of life- a product of your life- with you, and see these events unfold with ultrasound.

Fetal Development
Creating a new life is a spectacular miracle. Seeing that develop with ultrasound and sharing it with you is a truly an honor for us.
(This is a long video with commercial breaks, but well worth watching)
Prenatal Testing- 12 week Nuchal Translucency (NT)
Tests for fetal chromosome abnormalities include (Also see Testing):
- Nuchal transluency (NT) screen, with or without serum screen (PAPP-A and HCG). Nuchal translucency is measurement of the small amount of fluid seen behind the neck in all fetuses at 11-14 weeks. The amount of fluid tends to be larger in fetuses with Down syndrome and other major chromosome abnormalities. This test is usually performed together with a blood test measuring human chorionic gonadotropin (HCG), which tends to be increased in affected fetuses, and plasma associated protein A (PAPPA) which tends to be decreased in affected fetuses with Down syndrome. This test is offered as standard through the NHS. It can detect approximately 85% of major chromosome abnormalities like fetal Down syndrome, but there is also at least a 5-7% chance of a false positive result, in which case genetic amniocentesis or CVS might be suggested.
- NIPT (non invasive prenatal testing) for fetal chromosome abnormalities. These tests directly analyze fetal DNA fragments circulating in the maternal circulation. They are highly accurate for detecting fetal trisomy 21 (Down Syndrome) and other major chromosome abnormalities. The advantage is both the higher detection rate (99.5%) and the very low false positive rate (less than 0.5%).

NT = nuchal translucency, NB = nasal bone.
Fetal Anatomy scan 18-22 weeks
A routine fetal anatomy ultrasound scan should be performed at 18-22 weeks for all patients. A high quality ultrasound performed at the right time in pregnancy remains the single best test to screen your baby for possible important birth defects. It is the only method to detect birth defects with normal chromosomes (like most heart defects, brain abnormalities, kidney abnormalities, clubfoot, cleft lip/ palate) and can detect associated abnormalities in most cases with major chromosome abnormalities. In fact, depending on what is looked for, ultrasound alone can detect approximately 80% of fetuses with fetal Down syndrome.
There should be four primary goals:
1. Confirm normal growth
2. Confirm normal fetal development of the major organs
- Brain
- Spine
- Heart
- Face
- Stomach
- Abdomen
- Abdominal wall
- Kidneys/ kidney regions
- Urinary bladder
- Extremities- arms, legs, hands, feet
3. Evaluate 'minor' findings that are most often normal common variants, but which can indicate a higher risk for an underlying condition
4. Evaluate everything else- the uterus, cervix, placenta, umbilical cord, and adnexa.

Fetal Abnormalities at 18-22 weeks
Fetal development doesn't always happen perfectly. I have written two large textbooks on the types of conditions we can see with ultrasound. Also see download on birth defects below. Most birth defects are isolated defects involving a single organ- for example a heart defect. Other common examples include cleft lip/ palate, or clubfoot. When isolated, these types of detects are usually associated with normal chromosomes.
Birth defects may involve any organ
- Brain
- Spine
- Heart
- Face
- Stomach
- Abdomen
- Abdominal wall
- Kidneys/ kidney regions
- Urinary bladder
- Extremities- arms, legs, hands, feet
Major birth defects involving more than one organ system usually indicates an underlying chromosome abnormality or syndromic condition.
'Minor' findings that are most often normal common variants, but which can indicate a higher risk for an underlying condition. An echogenrdiac focus is the most commonly encountered example. A single umbilcal artery (two vessel umbilical cord) is another example. This variation is found in 0.5% of all pregnancies. It is usually isolated in which case there is no additional risk of an underlying fetal condition or syndrome.
The Placenta
The Placenta
The placenta is a vital connecting organ between the maternal uterus and the fetus.
It supports the developing fetus, in utero, by supplying nutrients, eliminating waste products of the fetus and enabling gas exchange via the maternal blood supply. It forms from the same DNA that makes the baby, and in this way it might be considered the 'forgotten twin'. Many of the problems encountered in the 3rd trimester are due to problems with the placenta or the placental-uterine (baby-mother) interface.
In this section , we review the development of the placenta.
Pre-Implantation
The development of the placenta begins during implantation of the blastocyst from the same cells that make the embryo.
The 32-64 cell blastocyst contains two distinct differentiated embryonic cell types: the outer trophoblast cells and the inner cell mass. The trophoblast cells form the placenta. The inner cell mass forms the fetus and fetal membranes.
Implantation
On the 6th day, as the zona pellucida disintegrates, the blastocyst “hatches”, allowing for implantation to take place. The trophoblast cells interact with the endometrial decidual epithelia to enable the invasion into the maternal uterine cells. The embryo secretes proteases to allow deep invasion into the uterine stroma. Implantation is interstitial. Normal implantation occurs on the anterior or posterior wall of the body of the uterus. The most common ectopic implantation site is in the ampulla of the Fallopian tube.
On the 8th day of development, the trophoblast cells differentiate into the outer multinucleated syncytiotrophoblast which erodes maternal tissues by sending out projections and the inner mononucleated cytotrophoblast which is actively proliferating.
The syncytiotrophoblast is responsible for producing hormones such as the Human Chorionic Gonadotropin (hCG) by the second week which is used in pregnancy testing.
Post-Implantation
On day 9, lacunae or spaces form within the syncytiotrophoblast. The syncytiotrophoblast also erodes the maternal tissues allowing maternal blood from uterine spiral arteries to enter the lacunar network. Thus early uteroplacental circulation is established by the end of week 2.
Meanwhile, the cytotrophoblast begins to form primary chorionic villi or finger-like projections which penetrate and expand into the surrounding syncytiotrophoblast. In the 3rd week, extraembryonic mesoderm grows into these villi forming a core of loose connective tissue, at which point these structures are called secondary chorionic villi. By the end of the third week, embryonic vessels begin to form in the embryonic mesoderm of the secondary chorionic villi making them tertiary chorionic villi.
The cytotrophoblast cells from the tertiary villi grow towards the decidua basalis of the maternal uterus and spread across it to form a cytotrophoblastic shell. The villi that are connected to the decidua basalis through the cytotrophoblastic shell are known as anchoring villi. Villi growing outward within the intervillous space from the stem (anchoring) villi are called branching villi and provide surface area for exchange of metabolites between mother and fetus. Imagining the villi as tree-like projections can help visualise their structure.
Establishment of Circulation
Maternal spiral arteries undergo remodelling to produce low resistance, high blood flow conditions in order to meet the demands of the fetus. Cytotrophoblast cells invade the maternal spiral arteries and replace maternal endothelium. They undergo an epithelial to endothelial differentiation. This increases the diameter and reduces the resistance of the vessels.
Pre-eclampsia is a trophoblastic disorder related to failed or incomplete differentiation of cytotrophoblastic cells during the epithelial to endothelial transformation.
In the first trimester (0-13 weeks), the surface of the chorionic villi is formed by the syncytiotrophoblast. These cells rest on a layer of cytotrophoblastic cells that in turn cover a core of vascular mesoderm. Therefore, the placental barrier is relatively thick.
The surface area for exchange dramatically increases by full-term (27-40 weeks). The placental barrier is much thinner and the cytotrophoblast layer beneath the syncytiotrophoblast is lost.
The placental barrier is not a true barrier as it allows many substances to pass between the maternal and fetal circulations. Unfortunately, this means various drugs (e.g. heroin, cocaine) and viruses (e.g. CMV, rubella, measles) can enter the foetal circulation. As the maternal blood in the intervillous spaces is separated from the foetal blood by chorionic derivatives, the human placenta is known as the haemochorial type.
By the fourth month, the placenta has two components: the maternal portion i.e. the decidua basalis and the fetal portion i.e. the chorion frondosum. As the pregnancy advances, the chorion frondosum or the “bushy” chorion is formed as more villi develop on the embryonic pole. On the fetal surface, the placenta is covered by the chorionic plate; on the maternal side it is bordered by the decidua basalis of which the decidual plate is most intimately incorporated into the placenta.
During the fourth and fifth months, the decidua form decidual septa which project into the intervillous space but do not join the chorionic plate. These septa have a core of maternal tissue but are covered by a layer of syncytial cells. At all times there is a syncytial layer that separates maternal blood in intervillous lakes from foetal tissue of the villi. The septa divide the placenta into compartments called cotyledons. Cotyledons receive their blood supply through 80-100 spiral arteries that pierce the decidual plate.
Full-Term Placenta
Discoid in shape with a diameter of 15-25cm, approximately 3 cm thick and weighs about 500-600g.
At birth, it is torn from the uterine wall and around 30 minutes after the birth of the child it is expelled from the uterine cavity.
The maternal side will have 15-20 bulging areas which are the cotyledons, covered by a thin layer of decidua basalis. Intervillous lakes of the fully grown placenta contain approximately 150 mL of maternal blood, which is renewed 3-4 times per minute.
The foetal surface is covered by it’s chorionic plate. The chorionic vessels converge toward the umbilical cord. These are a number of large arteries and veins. The chorion is covered by a layer of amnion.The umbilical cord usually attaches in the middle of the placenta, perpendicular to it. A velamentous insertion may occur if the umbilical cord inserts outside of the placenta, but this is rare.
End of Pregnancy
The aim of the changes that occur to the placenta at the end of pregnancy is to reduce exchange between the maternal and foetal circulations. These changes are as follows:
- Increase in the fibrous tissue in the core of the villus
- Thickening of foetal capillary basement membranes
- Obliterative changes in small capillaries of the villi
- Deposition of fibrinoid on the surface of the villi in the junctional zone and in the chorionic plate.
Deposition of fibrinoid results in infarction of an intervillous lake or sometimes an entire cotyledon, which subsequently turns whitish in colour.
Cervix
The cervix is the 'plug' that holds the pregnancy in the uterus. We want it to hold the pregnancy in tight while you walk, run or exercise, but we want it to dilate to 10 cm to allow vaginal birth at term. Considering the weight of the pregnancy, that's a very special plug!!
The closed cervix should measure at least 30 mm at this time- and is often over 40 mm (Caution: a full urinary bladder or focal uterine contraction can elongate the perceived cervical length and make it appear longer than it really is).
A shortened cervix indicates a higher risk for preterm delivery with the highest risk of extreme prematurity for patients with cervical length of less than 20 mm. Read more about prematurity, prevention and treatment.
Cervical shortening and/ or dilatation usually does not occur until after 18 weeks, simply because there isn't enough weight from the pregnancy to test it before then. Any bleeding after 16-18 weeks should be taken seriously because it could indicate cervical shortening or dilatation.

3rd Trimester
Time for Growth and Maturation
The third trimester is the true time for incubation and growth. After 24 weeks, babies continue to grow in length at the same rate but now accelerate growth and maturation Your baby is formed, but needs to mature and grow, and get ready for the outside world. The primary issues to watch for are:
- Preterm delivery
- Pre-eclampsia/ hypertension (more common with first time mums)
- Growth delay
- Gestational diabetes
It's important to distinguish SGA (small for gestational age) babies from those with IUGR (intrauterine growth restriction). Most small babies are perfectly healthy. In fact, if we knew that all small babies are healthy, both patients and doctors might be saying the smaller the better (at least in terms of birth itself). Of course we don't say that, but it's to emphasize that size is not the issue; health is the issue (that's true after birth as well!). Most small babies are small because they were meant to be that way- it's programmed into their DNA, usually based on parent size (maternal contribution probably more important than paternal before birth). True IUGR implies that the baby is small because it's not getting enough of what it needs. A mother's diet is almost always sufficient for baby's growth, so that's not the issue. What then? The answer of course lies with the placenta- the mediator between mother and baby. If the placenta is not functioning properly then baby may not grow as is it should. In addition to smaller baby size, other clues to placental dysfunction include
- decreased amniotic fluid
- Abnormal Doppler studies (umbilical, middle cerebral artery)
- Decreased fetal activity (late stages)
This and other potential complications of pregnancy are covered in a separate post.

Preeclampsia
Preeclampsia
Partly from the BMJ
Pre-eclampsia is a common disorder that is more common with first pregnancies. The underlying etiology is thought to be defective trophoblastic invasion as illustrated above. This important process is, at least in part, modulated by vitamin D. Defective trophoblastic invasion relates to the fetal-maternal interface. This can be considered as part of 'placental related' complications. Placental related complications usually manifest during the 3rd trimester and also include growth restriction, placental abruption, preterm birth even stillbirth. Recurrent or persistent bleeding in the first half of pregnancy probably also reflects placental dysfunction and can indicate an increased risk later in pregnancy.
Although the stage is set early, clinical presentation doesn't usually occur until the 3rd trimester when the placenta becomes more stressed. Symptoms are highly variable but hypertension and proteinuria are usually seen. These systemic signs arise from soluble factors released from the placenta as a result of a response to stress of syncytiotrophoblast. There are two sub-types: early and late onset pre-eclampsia, with others almost certainly yet to be identified. Early onset pre-eclampsia arises owing to defective placentation, whilst late onset pre-eclampsia may center around interactions between normal senescence of the placenta and a maternal genetic predisposition to cardiovascular and metabolic disease. The causes, placental and maternal, vary among individuals. Recent research has focused on placental-uterine interactions in early pregnancy. The aim now is to translate these findings into new ways to predict, prevent, and treat pre-eclampsia.
Pre-eclampsia is usually symptomless, making the syndrome hard to predict. Symptoms such as epigastric pain or severe headache frequently herald a terminal crisis, for example eclampsia or the HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome, which requires prompt termination of pregnancy. Screening of well women for the early stages of pre-eclampsia has been highly successful in limiting maternal and perinatal problems. Antenatal care is based on predictions of the chances of pre-eclampsia developing before the next screening tests are due.
In high income countries this situation has changed substantially. Impending or active pre-eclampsia can be detected by circulating biomarkers or Doppler ultrasound assessment of the uteroplacental circulation. This can be useful for the early onset but not late onset form of the syndrome. Circulating biomarkers may be placental or maternal. New hypertension or proteinuria are maternal markers of endothelial activation, whereas placental syncytiotrophoblast factors are further upstream in the pathophysiology and likely to be more precise. An increased ratio of sFlt-1/PlGF is a good marker of the placental component of pre-eclampsia, and of fetal growth restriction induced by placental malperfusion
The ability to exclude pre-eclampsia is also important, and before 35 weeks the PlGF value can rule out the need for delivery within the next two weeks with 98% probability. When combined in the sFlt-1/PlGF ratio, this increases to a probability of more than 99% within the next week. As with uterine artery Doppler assessment, the ratio does not predict late onset pre-eclampsia well. Ideally, diagnosis should be made early in pregnancy, when interventions could begin before the clinical features are manifest. Combinations of demographic and clinical factors with maternal blood pressure, uterine artery Doppler measurements, and blood biomarker assessments factors have been assembled to improve predictive efficiency. A version of enhanced first trimester screening has been used in a trial of prophylactic low dose aspirin with encouraging results, identifying primarily early onset pre-eclampsia.
One third of infants of pre-eclamptic pregnancies are growth restricted and have the same increased risk of obesity, diabetes, hypertension, and other chronic diseases as other growth restricted infants.
At present, the early and late onset forms are the probable extremes of a spectrum. The early onset form is predominantly due to defective placentation during the first few weeks of pregnancy, and shares a common initiating pathophysiology to other disorders of placentation, especially FGR. Various strands of evidence indicate that the level of placental insult is greater in pre-eclampsia than in FGR, stimulating the release of a heavier burden of placental pro-inflammatory factors. The concentration of these factors, and their interactions with the maternal constitution, will determine the inflammatory response that distinguishes between the two conditions. By contrast, late onset pre-eclampsia appears to be driven by oxidative changes in the placenta induced by a progressive mismatch between maternal perfusion and feto-placental demands, coupled with a maternal predisposition to cardiovascular disease.
Vitamin D and 3rd Trimester of Pregnancy
Vitamin D is really an essential prohormone your body makes every day. But due to modern lifestyle, we don't usually make enough. It plays a critical role in many processes including bone formation, calcium metabolism, placental development controlled placental invasion and brain development. In addition to the effects in adults (see figure right), compelling new evidence strongly indicates that vtamin has D been shown to reduce the risk of a variety of pregnancy related complications, many of which can be traced to placental function.
- prematurity (preterm birth)
- preeclampsia and fetal growth restriction
- gestational diabetes
- possibly reduce the risk of miscarriage
- contributes to the regulation of placental inflammation.
- reduce the risk of autism, both in animal and human studies
Importantly vitamin D therapy has also been shown to improve symptoms in children with autism and reduce the risk of autism. The benefit of vitamin D may be related to the duration of supplementation with some studies showing benefit when vitamin D is started a year before pregnancy.
There is now strong and compelling evidence that maintaining adequate vitamin D levels, > 40 ng/ ml, has many health benefits for both the mother and the baby.
Vitamin D deficiency in pregnant women has been shown to be associated with increased risk for pregnancy complications . These include preeclampsia , fetal growth restriction, small-for-gestational-age fetus , bacterial vaginosis and gestational diabetes mellitus. Maternal vitamin D deficiency has also been linked to adverse effects in offspring, including reduced bone density and childhood rickets, as well as increased risk of asthma and schizophrenia. Low levels of vitamin D appear to contribute to the risk of autism, according to a study published in Molecular Psychiatry: According to the researchers, women with low vitamin D levels at 20 weeks of pregnancy are more likely to have children with symptoms of autism. Children born to women who had low blood levels of vitamin D while pregnant more than double their risk of autism according to a study of more than 4,000 children in the Netherlands.
Vitamin D supplementation requirements appear to be greater than usually recommended, especially for patients found to have low vitamin D levels or for pregnancy. These patients should take at least 4000 IU/ml- or about 10 x the traditional recommendation of 400. It has been shown that maintaining a vitamin D level of >40 IU/ ml can reduce the risk of preterm delivery, and preeclampsia and improve pregnancy outcomes!

Common Problems
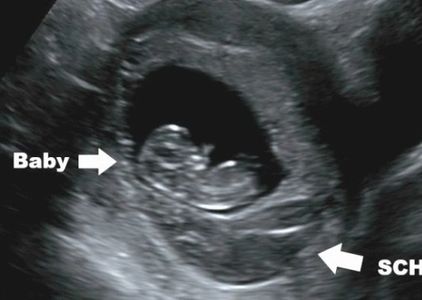
BLEEDING IN PREGNANCY
BLEEDING IN PREGNANCY
Many women will have some bleeding during pregnancy, especially in the first half of pregnancy. Here is a list of common reasons for bleeding during pregnancy, based on weeks of gestation.
To 13 Weeks
Bleeding is common. It almost always arises from the placenta or placental margin (occasionally from the cervix). Placental bleeding may or may not form a subchorionic hemorrhage (SCH). Subchorionic hemorrhage may be very small and hard to visualize, or very large- even larger than the pregnancy. Older blood appears dark by ultrasound, more recent bleeding appears echogenic (brighter).
What causes placental bleeding and subchorionic hemorrhage? Remember the placenta (which arises from baby) is attached to the uterus (you). Think of it as a special kind of glue. We want the glue to work well throughout pregnancy, but we want it to become unglued so the placenta can also deliver soon after birth of the baby. In the mean time, the uterus is growing, the placenta is growing, and the uterus has lots of contractions to help it grow. So...we are asking a lot from that special glue, and it's not surprising it can become unglued around the edges. The placenta is very vascular so any little bit of 'ungluing' is likely to result in bleeding.
Sometimes bleeding is a sign of miscarriage so it's best to make sure baby is ok with an ultrasound. If baby is ok, then there is little reason to worry, regardless.
From 13 Weeks to 22 Weeks
The same reasons for bleeding in the first 13 weeks of pregnancy can cause bleeding after 13 weeks, too. The cervix can still bleed, although less commonly. Bleeding from the subchorionic hematoma is usually dark, and is old blood, rather than new bleeding. NEW onset vaginal bleeding at this time in pregnancy should be evaluated with examination, including a speculum exam to see, and fingers to feel. Bleeding can be from the cervix opening up too soon.
From 22 Weeks to 34 Weeks
Cervix infection and irritation can cause bleeding at any time in pregnancy. More concerning is bleeding from placenta previa or the early onset of labor. Most patients will know about a low placenta from the ultrasound done at 20 weeks. Labor, or cervix dilation, can only be diagnosed by examination, best with finger exam of the cervix, sometimes by ultrasound. Bleeding with pain is most commonly from labor, but can also be caused by early detachment of the placenta, called abruption. Placental abruption usually has heavier bleeding, more pain, and more constant pain. Bleeding from placenta previa is usually painless. In all cases of bleeding at this time in pregnancy,
Between 34 and 37 Weeks
The most common cause of bleeding at this time in pregnancy is Premature Labor. As the cervix gets ready for labor, as it dilates, it can bleed. The dilation causes a mucousy discharge, which can be tinged red or brown, depending on how fresh the blood is. If the water bag breaks as the cervix dilates, fluid coming from the vagina can be red, mimicking heavy bleeding. We rarely stop a labor which starts after 34 weeks. Many babies born at this time in pregnancy will need extra medical attention at birth, so hospital evaluation is best. Placenta previa and placental abruption can also cause bleeding at this time.
After 37 Weeks
The most common cause of bleeding at this time in pregnancy is labor. The bleeding can be like the onset of a period. Light bleeding, without pain, can be managed just like the onset of labor. Heavier bleeding can still be labor, but patients should go in for evaluation sooner.
Mural nodules are very suspicious
Downloads
Vitamin D and pregnancy (pdf)
DownloadFact-Sheet-Vitamin-D-September-2017 (pdf)
DownloadEctopic Pregnancy ACOG interim update (pdf)
DownloadVitamin D and preterm birth (pdf)
DownloadVitamin D, the placenta and early pregnancy (pdf)
DownloadVitamin D requirements during pregnancy (pdf)
Downloadvitamn D and pregnancy complications (pdf)
DownloadVitamin D lowers risk of PTB 2017 (pdf)
Download